K C Al Si and Ba. Note that helium has the highest ionization energy of all the elements.

Lesson 13a Periodic Trends Chemistry Quiz Quizizz
Heres a ten question multiple choice quiz you can take to see if you understand important features about how the periodic table is organized and how it can be used.
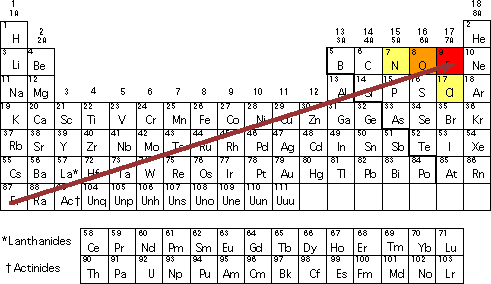
. An atoms electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The periodic table is a systematic way of organizing information about the elements. From left to right across a period of elements electronegativity increases.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. The higher the associated electronegativity the. Long Answer Type Questions.
Its monatomic form H is the most abundant chemical substance in the Universe constituting. Electronegativity symbolized as χ is the tendency for an atom of a given chemical element to attract shared electrons or electron density when forming a chemical bond. Iii Less metallic in character because on moving across a period metallic nature decreases.
Use Mendeleevs periodic table to predict the formula for the oxides of the following elements. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Ii Boron has the highest electronegativity because electronegativity decreases down a group.
The following series of problems reviews general understanding of the aforementioned material.

0 Comments